Reclaim your life from the impacts of UUI with a treatment designed to fit your lifestyle.
Learn More

life,
uncompromised.
Learn Moreyour challenge
is our purpose.
When? You don’t know when, but often. It’s interruptive. It’s embarrassing. It makes life, well, frustrating. At Neuspera, we understand your challenge with Urinary Urge Incontinence (UUI), a symptom of Overactive Bladder (OAB) affecting 1 in 5 women in the U.S. Our integrated sacral neuromodulation (iSNM) system is a smart solution designed to fit seamlessly into your daily life. Learn more about our clinical study.
Urinary Urge Incontinence
The Neuspera Integrated Sacral Neuromodulation (iSNM) System offers patients with urinary urge incontinence (UUI) a smarter, integrated alternative to traditional SNM.
For Patients
For Physicians
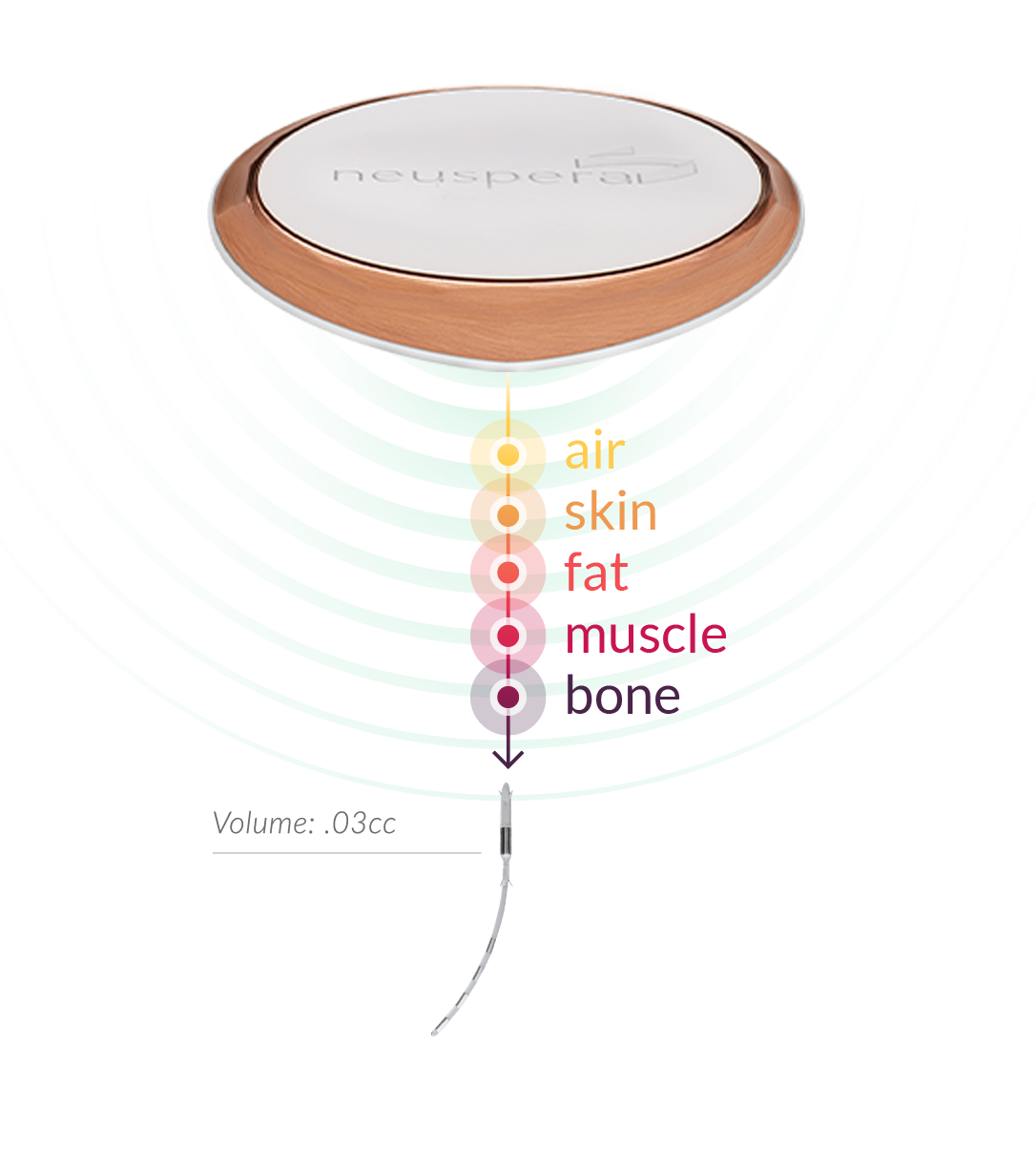
Our Technology
At the core of our technology platform is a smart neurostimulator that is implanted near the sacral nerve to deliver sacral neuromodulation (SNM) therapy for the treatment of urinary urge incontinence (UUI), a symptom of overactive bladder. The therapy is delivered through a discreet external battery worn daily. It charges wirelessly, like a smartphone, and avoids the complications of implanted batteries.

Patient-Centered Therapy
Next-Gen Integrated Device for UUI
About Neuspera Medical
Neuspera Medical, Inc. is a leader in advanced, directionally adaptive energy systems that power medical implants deep within the body. The company is introducing a new class of neurostimulation technology, starting with the first integrated sacral neuromodulation (iSNM) system for urinary urge incontinence (UUI), approved by the FDA in June 2025.
Learn More

